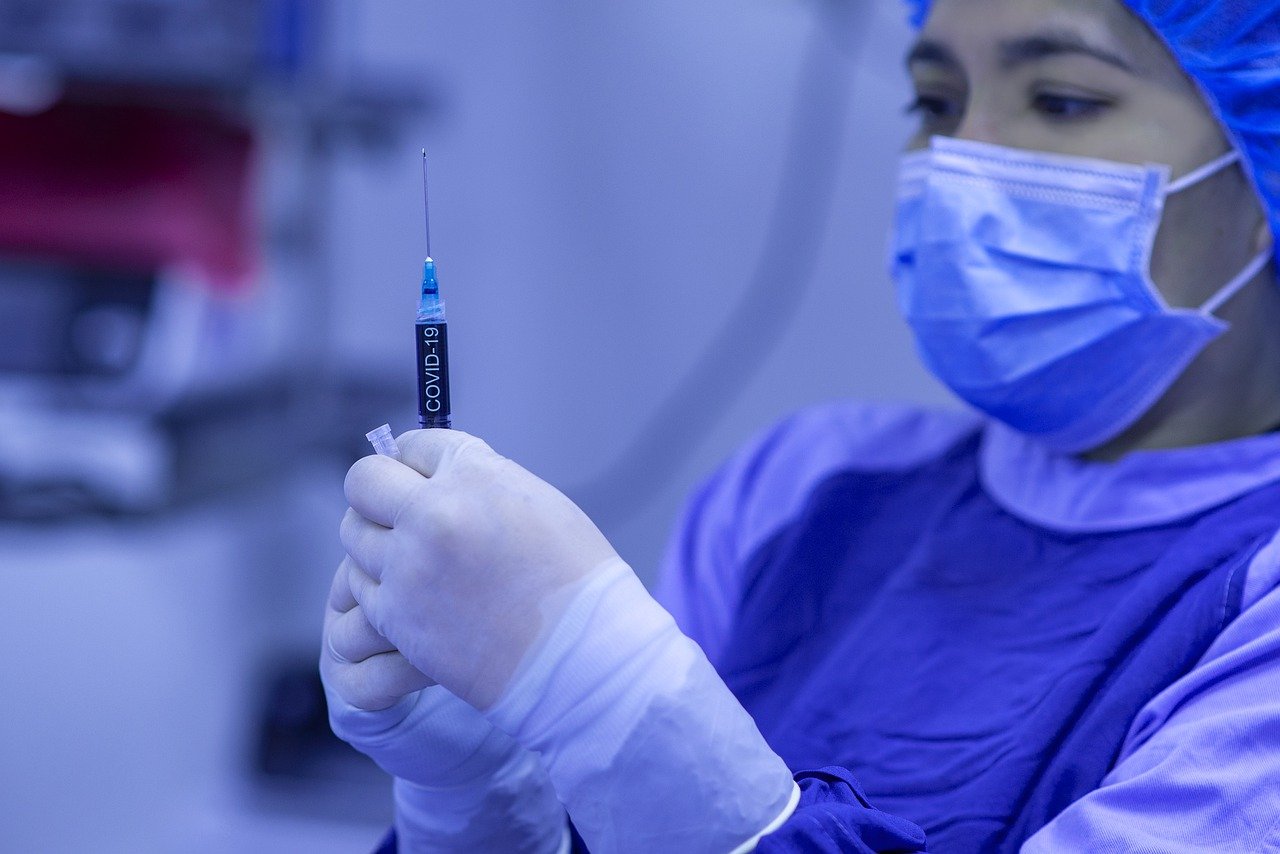
Pfizer’s coronavirus vaccine for children under 5 years of age could be available by the end of February. According to a plan that would lead to the possible authorization of the vaccine schedule in children in the coming weeks, which has been accessed by the newspaper ‘The Washington Post’.
Pfizer’s vaccine for children under 5 could be available by end of February
Pfizer and its partner, BioNTech, are expected to submit an emergency use authorization application for this new version of the Covid vaccine to the U.S. Food and Drug Administration as early as Tuesday. The drug can be administered to children between the ages of 6 months and 5 years, making it the first to come to market for that age group.
In addition, the Food and Drug Administration urged companies to submit the application so that regulators could begin reviewing data for two doses for that age range.
In recent months, companies have been testing a third dose, following results showing that the full vaccine schedule (two doses in children) generated less immunity than expected. But data on a third jab will not be available until at least the end of March. Once that information is submitted, regulators are expected to authorize a third dose of the pediatric vaccine.